The Fascinating World of pH: From Acidity and Alkalinity
- Kiera Castellon
- Nov 5, 2024
- 3 min read

Have you ever wondered why some substances taste sour while others taste bitter? Or why your skin feels slippery after washing with soap? The answer lies in the magical world of pH — a concept that governs the acidity or alkalinity of substances all around us. In this blog post, we'll explore the basics of pH, from its definition to its practical applications in everyday life.
Understanding pH: What is it, Anyway?
Let's start at the beginning. pH is a measure of how acidic or alkaline a substance is. It's a scale that ranges from 0 to 14, with 7 being neutral. Substances with a pH below 7 are considered acidic, while those with a pH above 7 are alkaline (or basic). This simple scale helps us understand the properties of different substances and how they interact with each other.
Acids have a pH less than 7
Neutral solutions have a pH of 7
Alkalis have a pH greater than 7

The Power of Hydrogen Ions: Unveiling the Science Behind pH
But what exactly determines whether a substance is acidic or alkaline? The answer lies in the concentration of hydrogen ions (H⁺) in the substance. In acidic substances, there's a high concentration of hydrogen ions, while in alkaline substances, there's a low concentration. These hydrogen ions play a crucial role in chemical reactions and can affect everything from taste and texture to the effectiveness of cleaning products.
Everyday Examples of pH in Action
pH isn't just a concept confined to the laboratory—it's all around us, shaping our everyday experiences in countless ways. Here are just a few examples of pH in action:
Lemon Juice (pH ≈ 2): Ever puckered up after taking a sip of lemon juice? That's because lemon juice is highly acidic, with a pH of around 2. Its acidity gives it that tart taste that makes it perfect for adding a zing to your favorite dishes and drinks.
Baking Soda (pH ≈ 9): On the opposite end of the spectrum, baking soda is alkaline, with a pH of around 9. Its alkalinity makes it a powerful cleaning agent, capable of breaking down grease and grime on surfaces and neutralizing odors.
Rainwater (pH ≈ 5.6-7): Did you know that rainwater isn't completely neutral? It's slightly acidic, with a pH ranging from 5.6 to 7, due to the presence of carbon dioxide in the atmosphere, which forms carbonic acid when it dissolves in rainwater.

The Role of pH in Biology and Health
pH isn't just important in chemistry—it's also essential for maintaining balance in living organisms. In the human body, for example, pH plays a critical role in regulating bodily functions and keeping us healthy. Our blood, for instance, has a narrow pH range of around 7.35 to 7.45, and even a slight deviation can have serious consequences. Disorders like acidosis (excess acidity) or alkalosis (excess alkalinity) can disrupt cellular function and lead to health problems.
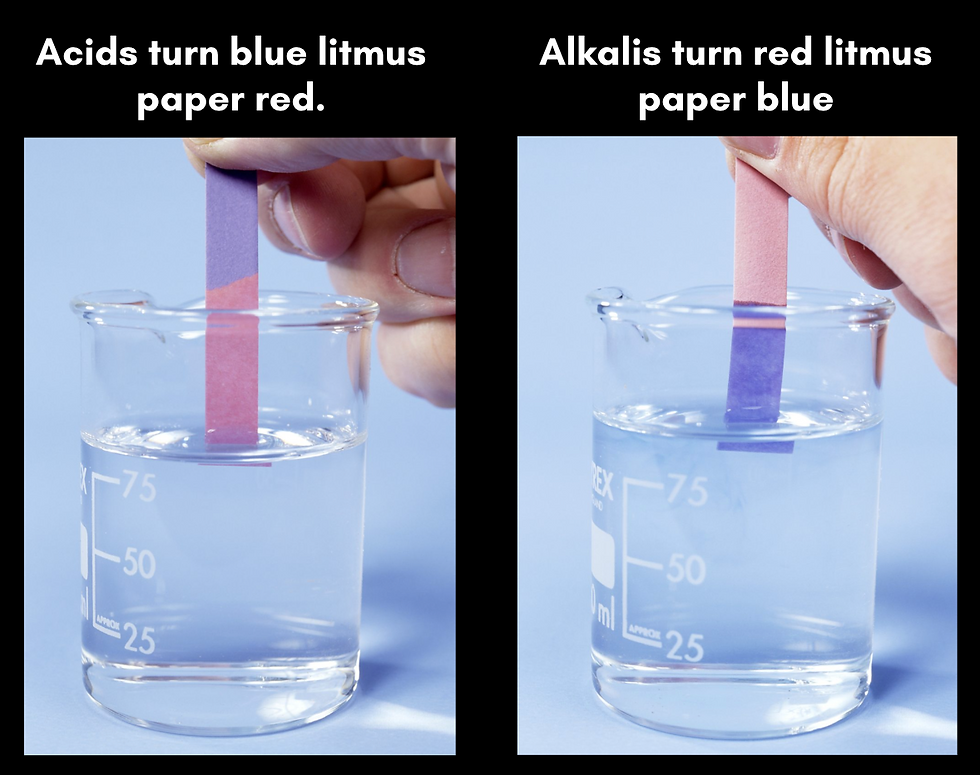
Litmus Test
Litmus indicator solution turns red in acidic solutions and blue in alkaline solutions. It turns purple in neutral solutions.
Navigating the pH Scale
In conclusion, pH is a powerful concept that governs the acidity and alkalinity of substances, shaping our world in countless ways. Whether you're enjoying a refreshing glass of orange juice, scrubbing away dirt with soap, or maintaining the delicate balance of your body's internal environment, pH is there, quietly influencing the world around us. So the next time you encounter a sour lemon or a slippery soap bubble, remember the invisible force at work—the power of pH!




Comments